Research Overview
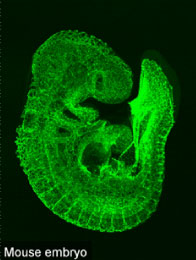 Fig. 1
Fig. 1
In mammalian development, a vascular network is formed throughout the body to meet the tissue requirements for oxygen and nutrients. Three major processes necessary to form a complete vascular network are vasculogenesis, angiogenesis and vascular remodeling. Vasculogenesis denotes de novo blood vessel formation, in which vascular precursor cells (angioblasts) migrate to sites of vascularization, differentiate into endothelial cells, and coalesce to form the initial vascular plexus. Angiogenesis refers to the budding of new capillary branches from existing blood vessels, while vascular remodeling describes a later phase when a formed vessel increases its luminal diameter in response to increased blood flow and acquires artery, vein or capillary identity. Once these three processes are completed during postnatal development, adult vasculature is stable and rarely proliferates under physiological conditions. Our research is aimed at uncovering the cellular and molecular mechanisms of vascular development basically using mice (Fig. 1). We are particularly interested in the shape of the vascular network which considerably differs from organ to organ, and in the acquisition mechanism of such diversity. In this mean, our research mainly relies on classical histological analyses. All of the members in my laboratory are always bear in mind “Eye-friendly research”. The achievement of our research, though looks very basic, may lead to discovery of novel therapeutic strategy against human neovascular and ischemic diseases.
Project 1: Heterogeneity and diversity of the tissue vascular patterning
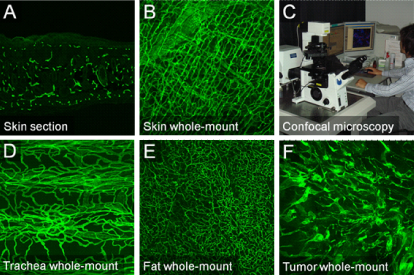 Fig. 2
Fig. 2
In our research, we assume it’s very important to analyze blood vessels in their original status as much as possible. For visualization of vascular structures, immunohistochemistry on tissue sections has long been used. However, in sections, we can see transverse planes of blood vessels and the resultant information is rather limited (Fig. 2A). Therefore, we have been trying to visualize the 3-D structures of blood vessels distributed in adult tissues by the whole-mount staining which has been used on mouse embryos. So far, after multiple trials and errors utilizing confocal microscopy (Fig. 2C), we have got images of rather good quality without any clearing agents (Fig. 2B). Not only blood vessels in normal tissues, but those in tumors were successfully visualized (Fig. 2D-F). We can clearly see that the vascular patterning totally differs from organ to organ. We are particularly interested in its morphological diversity and developmental dynamics during its establishment.
Project 2: Oxygen in the neuro-vascular interaction
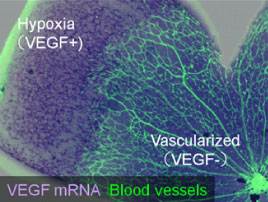 Fig. 3
Fig. 3
An oxygen supply, provided by the vascular network, is indispensable for tissue homeostasis throughout the body. During vascular development, endothelial cells interact with cells of other lineages, which exist in tissues before vascularization. Accumulating evidence shows that neuronal lineage cells contribute significantly to the formation of the vascular network. In the developing skin, for example, local signals such as vascular endothelial growth factor (VEGF), provided by organized sensory nerve fibers, define the pattern of blood vessel branching and arterial differentiation (Mukouyama et al., 2002). Our research is investigating the oxygen sensing mechanism related to the neurovascular crosstalk, in particular using the murine retina model In the developing retina, VEGF is abundantly expressed in hypoxic astrocytes and promotes vascular growth into the avascular area. When the area is vascularized and provided with oxygen, the VEGF expression is halted (Fig. 3). Such a reciprocal feedback between astrocytes and endothelial cells enables directional vascular growth and ensures a proper amount of blood vessels. We further investigated the role of retinal progenitor cells (RPCs), counterparts of neural stem/progenitor cells in the brain, in this machinery. The results indicate an exquisite interaction among RPCs, astrocytes, and endothelial cells is required for this hypoxia-driven vascular growth. (Kubota et al J Clin Invest 2008; Kurihara et al Development 2010; Nakamura-Ishizu et al Dev Biol 2012).
Project 3: Immune cells in angiogenesis
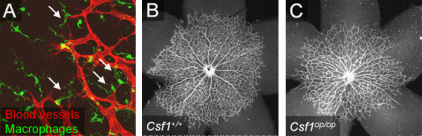 Fig. 4
Fig. 4
Macrophages are white blood cells produced through colony stimulating factor-1 (CSF-1)-dependent differentiation of monocytes. They function in both non-specific (innate immunity) and specific (adaptive immunity) defense mechanisms through phagocytosis of cellular debris and pathogens. Recent our and others’ evidence demonstrates that macrophages are also involved in vascular development by promoting angiogenic anastomosis (Kubota et al J Exp Med 2009; Fantin et al 2010; Rymo et al 2011). Tissue macrophages are largely located between two endothelial tip cells and are connecting their filopodia (Fig. 4A). Indeed, lack of macrophages in Csf1op/op mutant mice have reduced vessel branching in their retinas (Fig. 4B, C). In contrast to this proangiogenic action of macrophages, they are also known to induce endothelial cell death in hyaloid vessels and pupillary membranes, transient vascular networks seen in fetal eyes (Lang et al 1993; Lobov et al 2005). We are interested in how macrophages act on blood vessels, namely molecular effectors derived from them. Heterogeneity in macrophage populations may influence the diverse function of macrophages on angiogenesis.
Project 4: Mechanisms for tissue avascularity
Several tissues in our bodies lack blood vessels (e.g., cornea and intervertebral disks). Besides them, there are local areas where fewer blood vessels grow compared to surrounding areas (e.g. brain cortex and neuroretina). In many cases, paradoxically, such avascular or hypovascular areas highly express VEGF. This issue is, at least in the cornea, explained by the abundant expression of the soluble form of VEGF receptor 1 (sVEGFR1), which sequesters and inactivates VEGF proteins. Consequently, the VEGF level is very low in the corneal tissues, so the area is free from vascular invasion (Ambati et al 2006). Recently, we happened to find VEGFR2, a critical endothelial receptor for VEGF, is more abundantly expressed in retinal neurons than in endothelial cells. Utilizing this receptor, independently of the sequestration by the soluble form of VEGFR2, neurons endocytose and degrade VEGF proteins to limit vascular growth into the neuroretina (Okabe et al Cell 2014). We also found this neuronal endocytosis of VEGF turns on the switch of the programmed regression of hyaloid vessels (Yoshikawa et al., J Exp Med 2016) (Fig. 5). We are seeking the possibility that similar mechanism may underlie the tissue avascularity in tissues outside the central nervous system.
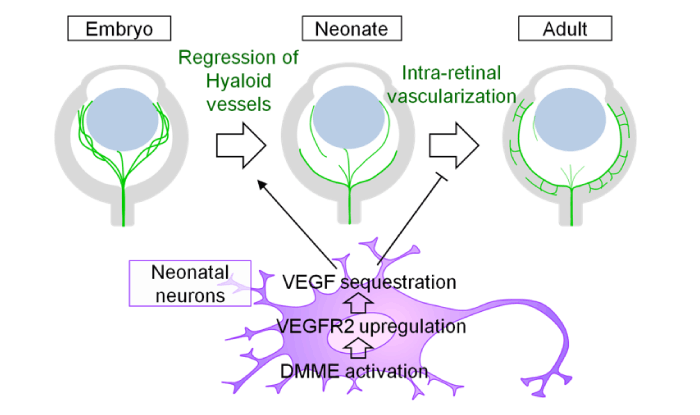 Fig. 5
Fig. 5
Project 5: Anatomy and development of lymphatic vessels
The two major circulatory systems of blood and lymphatic vasculature are discretely distributed throughout the body. While the former provides tissues with oxygen and nutrients, the latter drains the interstitial fluid from the tissue spaces to return it to the bloodstream, transports immune cells, absorbs dietary lipids, and clears waste from the brain. The structures of the two systems, the blood vessel (BV) and the lymphatic vessel (LV), are histologically similar but anatomically do not share the same lumen, except at the lymphovenous valve located near the venous angle, the final junction of collecting lymph ducts, and subclavian veins. LVs arise mostly from preexisting veins. The expression of Prox1, a master transcription factor controlling lymphatic specification, determines both the initiation of the identity as a lymphatic endothelial cell (LEC). However, the molecular mechanisms that ultimately maintain separation of these two circulatory systems have not been fully identified.
Recently, we found that genetic deficiency of Folliculin, a tumor suppressor, leads to misconnection of blood and lymphatic vessels in mice and humans. Absence of Folliculin results in the appearance of “lymphatic-biased venous endothelial cells” caused by ectopic expression of Prox1. Mechanistically, this phenotype is ascribed to nuclear translocation of the basic helix-loop-helix transcription factor Transcription Factor E3 (TFE3), binding to a regulatory element of Prox1, thereby enhancing its venous expression. Overall, these data demonstrate that Folliculin acts as a gatekeeper that maintains separation of blood and lymphatic vessels by limiting the plasticity of committed endothelial cells (Fig. 6) (Tai-Nagara et al., Nat Commun 2020).
The history of lymphatic vessel research is relatively short compared to that of blood vessels. In particular the structure of the lymphatic-venous junction located in the venous angle is poorly understood both in humans and mice. We are currently studying its anatomical structure and alteration during aging as well as the mechanisms for its maintenance utilizing our whole-mount technology.
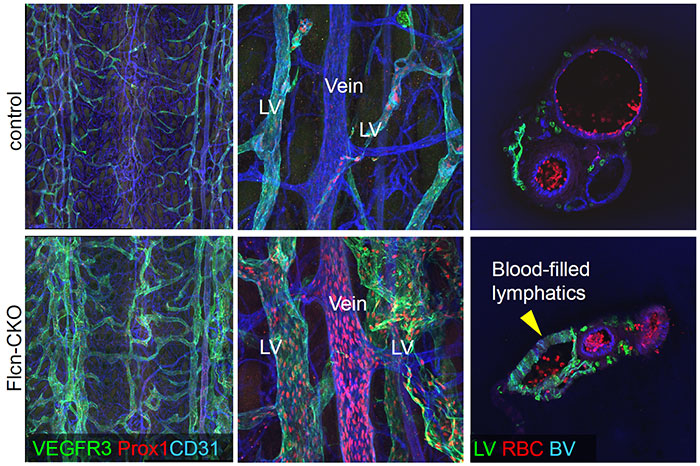 Fig. 6
Fig. 6

